Welcome to RYBELSUS® (semaglutide tablets)

This is not a real patient and is for illustrative purposes only.
Welcome to the RYBELSUS® UK Patient Website
Welcome! You are here either because you have been prescribed RYBELSUS® yourself, or you are a carer of someone who has been prescribed the medication.
This website has been developed by Novo Nordisk UK to provide a range of resources to support your use of RYBELSUS®.
Please note RYBELSUS® is undergoing a transition which will affect your RYBELSUS® tablets. A new formulation of RYBELSUS® is replacing the previous formulation. The new tablets are smaller in size and offer equivalent efficacy, safety and method of administration as your previous tablets.
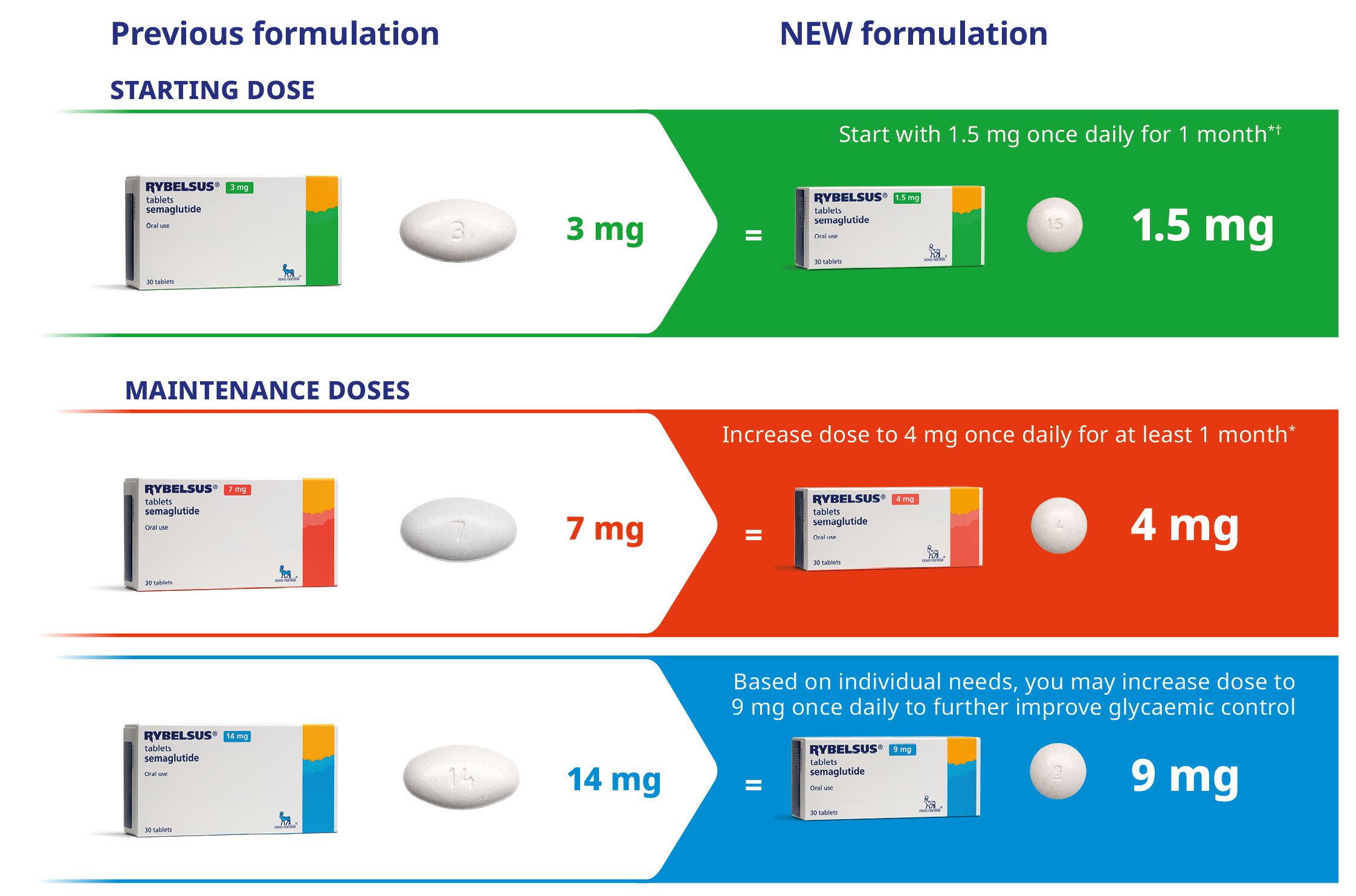
*1 month = 30 days
† 1.5 mg is not a maintenance dose and is intended to help patients start treatment
The pack shots and tablet images are for illustrative purposes only and do not reflect the actual size of the pack or tablet.
This information does not replace the Patient Information Leaflet, which you are advised to read in full. It is not intended as a substitute for clinical advice provided by your healthcare professional. Please contact your healthcare professional if you have any questions about your treatment and for clinical advice.
For information on reporting side effects please see the bottom of this page.
In the links below you can access the RYBELSUS® Patient Information Leaflets, which can be found on the electronic Medicines Compendium (eMC):
RYBELSUS® previous formulation
- RYBELSUS 3 mg – Patient Information Leaflet
- RYBELSUS 7 mg – Patient Information Leaflet
- RYBELSUS 14 mg – Patient Information Leaflet
RYBELSUS® new formulation
Why have you been prescribed RYBELSUS®?
Having type 2 diabetes means that managing your blood sugar levels within a normal range is very important.
You and your healthcare professional have decided that RYBELSUS® is the appropriate medication to help you do this.
What is RYBELSUS® and what is it used for?
RYBELSUS® is a medicine containing the active substance semaglutide, which is used to lower blood glucose (sugar) levels in adults (18 years and older) with type 2 diabetes when diet and exercise is not enough. It can be used on its own when metformin (another medicine for diabetes) cannot be used, or in combination with other diabetes medicines, when the other medicines are not enough to control your blood sugar levels.
It is important that you continue with your diet and exercise plan as agreed with your doctor, pharmacist or nurse.
Resources to support you with your RYBELSUS® tablets
RYBELSUS® is undergoing a transition which will affect your RYBELSUS® tablets. A new formulation of RYBELSUS® is replacing the previous formulation.
We have developed materials to support you during the transition from the previous formulation of RYBELSUS® to the new formulation.
The new RYBELSUS® tablets have been improved to help your body absorb the medication more effectively than your previous RYBELSUS® tablets.
This means you can get equivalent results with a lower dose and a smaller tablet.
Learn more about the new formulation by accessing the RYBELSUS® patient transition guide here.
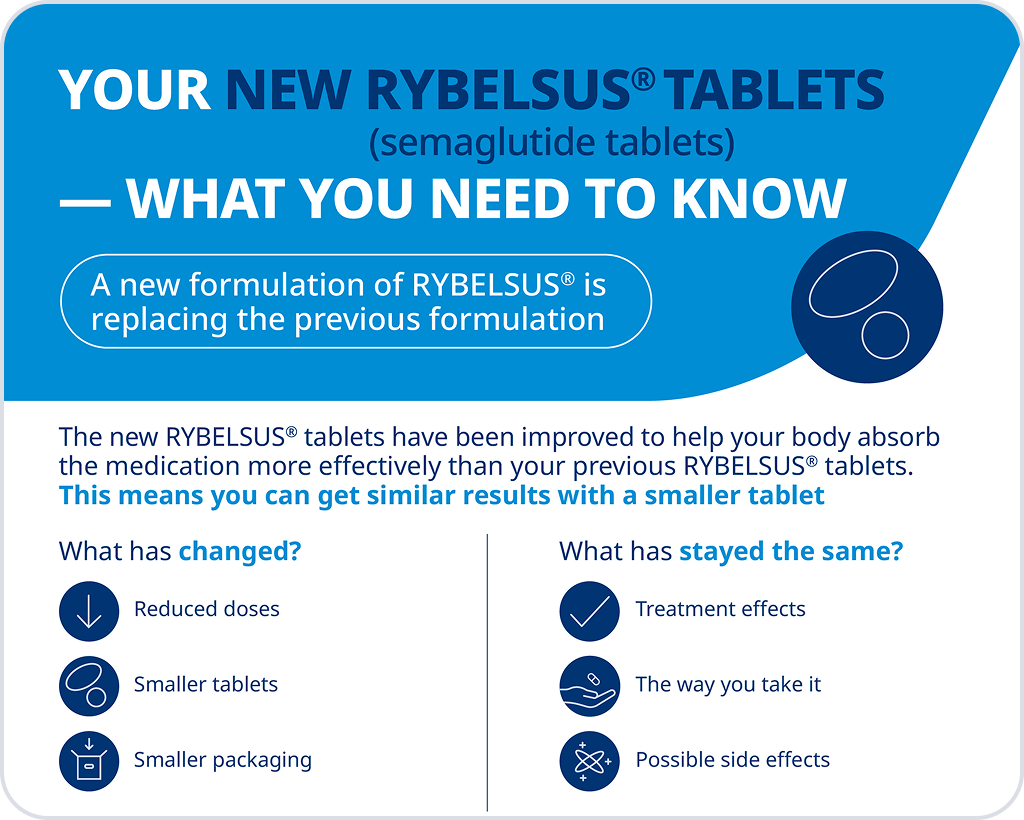
What you need to know about your NEW RYBELSUS® tablets
Watch this video guide to learn how to take your new RYBELSUS® tablets safely and effectively.
We have developed a digital booklet with you in mind to support you throughout your RYBELSUS® treatment. It will give you information about RYBELSUS®, an explanation of how RYBELSUS® works and some things to remember when using RYBELSUS®.
If there are any words in this digital booklet that are new or difficult to understand, please look at the section called 'Useful words to know.'
If you still have any questions or queries, please speak with your healthcare professional.

Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the Yellow Card Scheme
at https://yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Side effects should also be reported to Novo Nordisk Limited (Telephone Novo Nordisk Customer Care Centre 0800 023 2573).
Calls may be monitored for training purposes. By reporting side effects, you can help provide more information on the safety of this medicine.
See yellowcard.mhra.gov.uk for how to report side effects.